Moderna announced study showing Its COVID-19 vaccine maintained antibodies against variants of concern
On Aug. 12, 2021, Moderna announced the publication of new data on the durability of the Moderna COVID-19…

On Aug. 12, 2021, Moderna announced the publication of new data on the durability of the Moderna COVID-19…

On Aug. 12, 2021, Innovation Pharma released information regarding the status of its randomized, double-blind, placebo-controlled Phase 2…
On Aug. 10, 2021, the National Institutes of Health (NIH) announced that a pilot study had begun to…

On Aug. 10, 2021, Moderna announced a Memorandum of Understanding (MoU) with the government of Canada to build…

On Aug. 9, 2021, Moderna announced that the Therapeutic Goods Administration (TGA) had granted provisional registration to the…

On Aug. 9, 2021, Inovio Pharma announced that it had received regulatory allowance for two clinical trials investigating…

On Aug. 6, 2021, BD (Becton, Dickinson) and CerTest Biotec announced the CE mark for a molecular test…
On Aug. 5, 2021, Novavax announced preliminary data demonstrating that a single booster dose of its recombinant nanoparticle…

On Aug. 4, 2021, Regeneron announced that the New England Journal of Medicine (NEJM) had published positive detailed…
On Aug. 4, 2021, Inovio Pharma announced that the company had dosed the first Phase 2 trial subject…

On Aug. 4, 2021, Novavax announced that it had reached an agreement with the European Commission (EC) for…

On Aug. 3, 2021, CytoDyn announced that Brazil’s regulatory authority ANVISA (Agência Nacional de Vigilância Sanitária) has approved…

On Jul. 29, 2021, BOTOX(R) Allergan, an AbbVie company, announced that the U.S. Food and Drug Administration (FDA)…

On Jul. 29, 2021, Vaxart announced that it has shown for the first time in clinical trials that…
On Jul. 29, 2021, SIGA Technologies announced that it had entered into a collaboration with Oxford University in…

On Jul. 29, 2021, Cocrystal Pharma announced that its SARS-CoV-2 3CL protease lead CDI-45205 and several analogs showed…

On Jul. 28, 2021, GlaxoSmithKline and Vir Biotechnology announced they had signed a Joint Procurement Agreement with the…

On Jul. 27, 2021, Oragenics announced it had entered into a licensing agreement with the National Research Council…
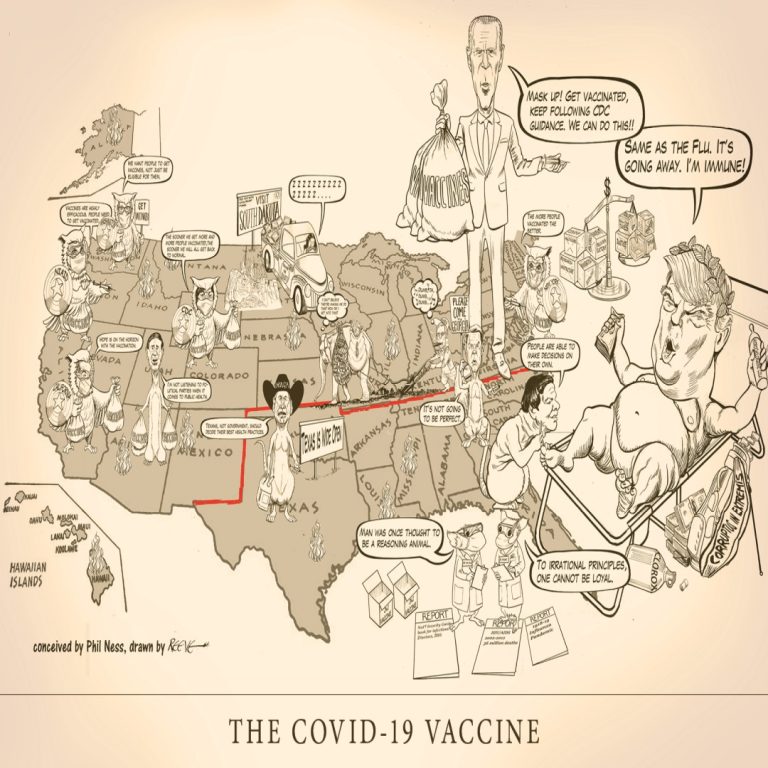
Cast of Characters: Donald Trump, President | Mike Pence, Vice President | Anthony S. Fauci, MD, Director, National…
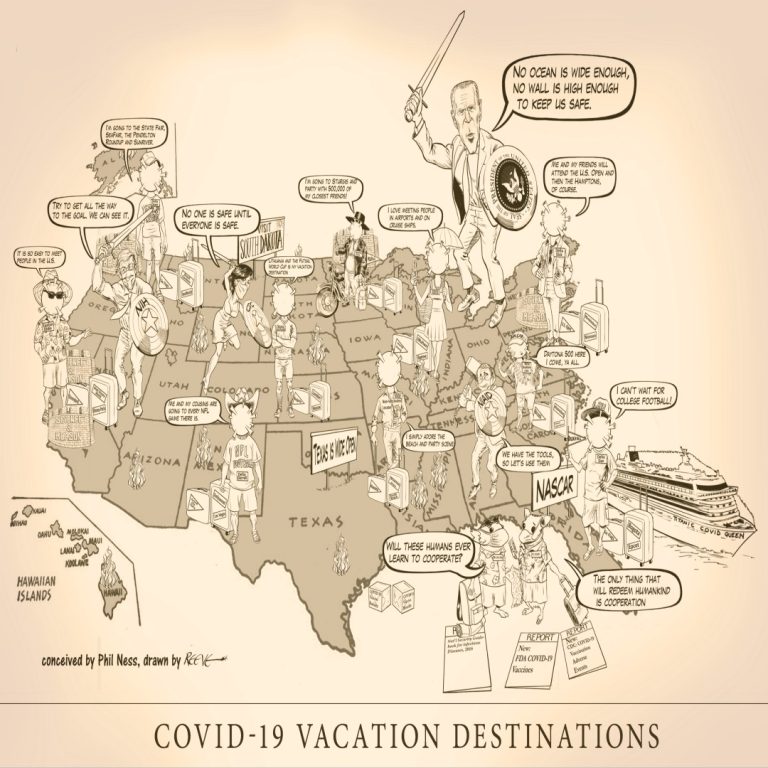
Planning a Summer vacation? Where to go? How about going to one of those large events with tens…

On Jul. 23, 2021, Moderna announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human…
On Jul. 22, 2021, Moderna announced a new supply agreement with Taiwan for 20 million doses of Moderna’s…

On Jul. 22, 2021, the National Institutes of Health announced it had renewed grants to seven regional centers…

On Jul. 20, 2021, Moderna announced that the Ministry of Health, Labour and Welfare of Japan (MHLW) and…

On Jul. 16, 2021, Pfizer and BioNTech announced that the U.S. Food and Drug Administration (FDA) had granted…
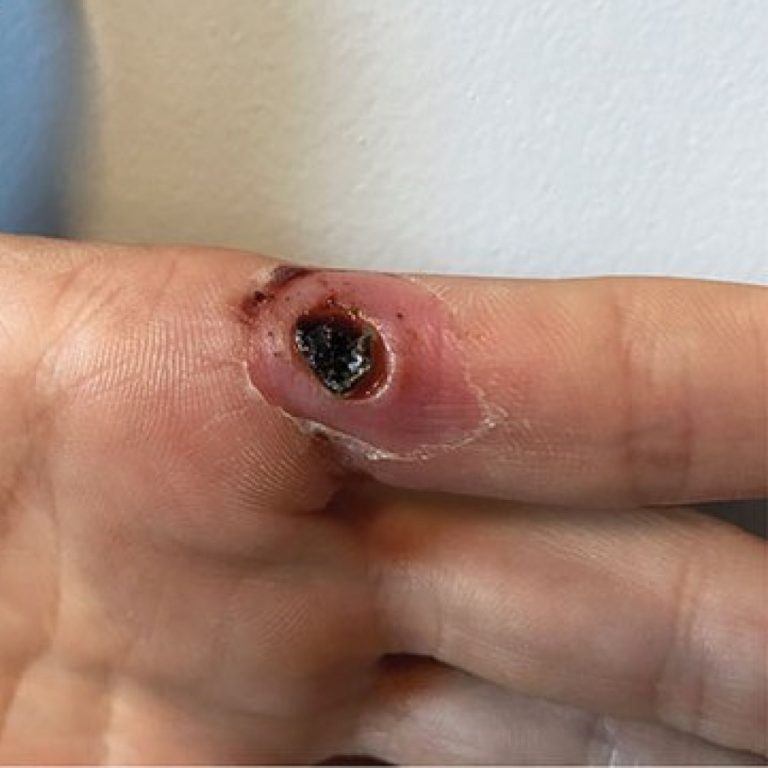
On Jul. 16, 2021, the U.S. Centers for Disease Control and Prevention (CDC) announced and the Texas Department…
On Jul. 16, 2021, Merck announced the U.S. Food and Drug Administration (FDA) had approved VAXNEUVANCE (Pneumococcal 15-valent…

On Jul. 12, 2021, Moderna announced a supply agreement with the government of Argentina for 20 million doses…

On Jul. 9, 2021, a federal jury convicted a timber thief who authorities said started a large forest…
On Jul. 8, 2021, the National Institutes of Health (NIH) reported that the overall cancer death rates continue…