CDC confirmed first human infection with flu virus from pigs during 2022
On Aug. 5, 2022, the Centers for Disease Control and Prevention (CDC) reported the first human infection with…

On Aug. 5, 2022, the Centers for Disease Control and Prevention (CDC) reported the first human infection with…
On Aug. 5, 2022, the U.S. Food and Drug Administration (FDA) approved Daiichi Sankyo’s Enhertu (fam-trastuzumab-deruxtecan-nxki), an IV…

On Aug. 4, 2022, Novavax announced the initiation of its Phase 2b/3 Hummingbird global clinical trial. The trial…

On Jul. 30, 2022, scientists announced they had sequenced the genome of a 6,000-year-old Citrullus seeds that revealed…

On Jul. 29, 2022, the U.S. Department of Health and Human Services (HHS), in collaboration with the U.S….
On Jul. 28, 2022, the Allen Institute announced a newly released dataset of 1.2M cells from brain donors…
On Jul. 28, 2022, the World Health Organization (WHO) released new guidelines for the use of long-acting injectable…

On Jul. 27, 2022, the Centers for Disease Control and Prevention (CDC) announced that it had identified for…
On Jul. 27, 2022, City of Hope announced that a 66-year-old man who was diagnosed with HIV in…

On Jul. 26, 2022, Novavax announced that the Australian Therapeutic Goods Agency had granted expanded approval for provisional…

On Jul. 26, 2022, Novavax announced that Nuvaxovid (NVX-CoV2373) COVID-19 vaccine had received expanded manufacturing and marketing approval…
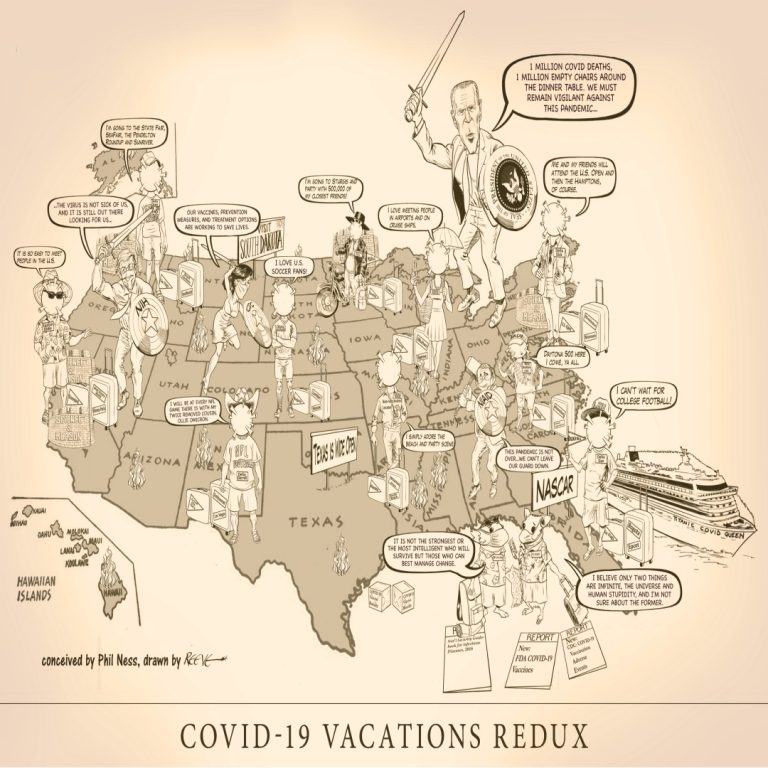
COVID-19 Vacations Redux iIlustrates once again the pits and perils vacation travelers face as they begin their Summer…

On Jul. 22, 2022, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…
On Jul. 22, 2022, Moderna announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human…

On Jul. 22, 2022, a team of scientists announced they had sequenced the genome of a 6,000-year-old citrullus…
On Jul. 21, 2022, The New York State Department of Health confirmed that a case of paralytic poliomyelitis…
On Jul. 19, 2022, Novavax announced that it had signed agreements with its partner, SK bioscience, for the…

On Jul. 19, 2022, Novavax announced that the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee…
On Jul. 18, 2022, Moderna announced that the Therapeutic Goods Administration (TGA) in Australia had granted provisional registration…

On Jul. 14, 2022, Moderna announced that Health Canada had approved the use of Moderna’s mRNA COVID-19 vaccine,…

On Jul. 13, 2022, Novavax announced that the European Commission (EC) had approved the expanded conditional marketing authorization…

On Jul. 13, 2022, Novavax announced that its COVID-19 Vaccine, Adjuvanted (NVX-CoV2373) had received emergency use authorization from…

On Jul. 11, 2022, Moderna announced new clinical data on its bivalent Omicron (BA.1) booster candidate, mRNA-1273.214. One…
On Jul. 11, 2022, the National Institute of Allergy and Infectious Diseases (NIAID) launched an early-stage clinical trial…

On Jul. 8, 2022, Pfizer and BioNTech announced that the companies had submitted a variation to the European…

On Jul. 8, 2022, the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service confirmed the state’s…

On Jul. 8, 2022, the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service (APHIS) confirmed the…

On Jul. 7, 2022, Novavax announced that the European Commission had approved a variation to allow SK bioscience…

On Jul. 5, 2022, Novavax announced that the European Commission (EC) had approved the expanded conditional marketing authorization…
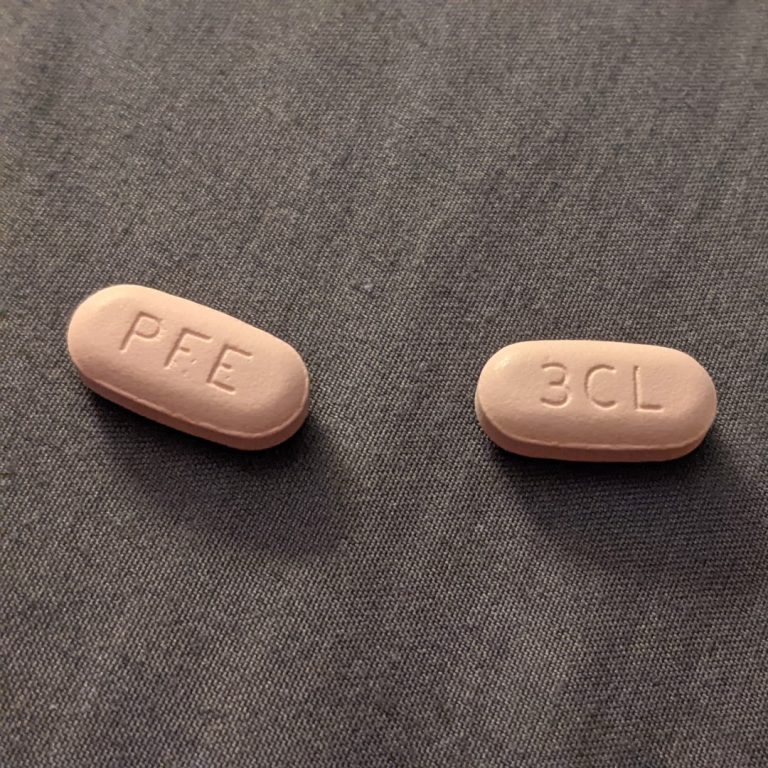
On Jun. 30 2022, Pfizer announced the submission of a New Drug Application (NDA) to the U.S. Food…