Pfizer and BioNTech received U.S. FDA Fast Track Designation for single-dose mRNA-based vaccine candidate against COVID-19 and influenza
On Dec. 9, 2022, Pfizer and BioNTech announced the companies had received Fast Track Designation from the U.S….

On Dec. 9, 2022, Pfizer and BioNTech announced the companies had received Fast Track Designation from the U.S….

On Dec. 9, 2022, the World Health Organization (WHO) announced that the first doses of one of the…
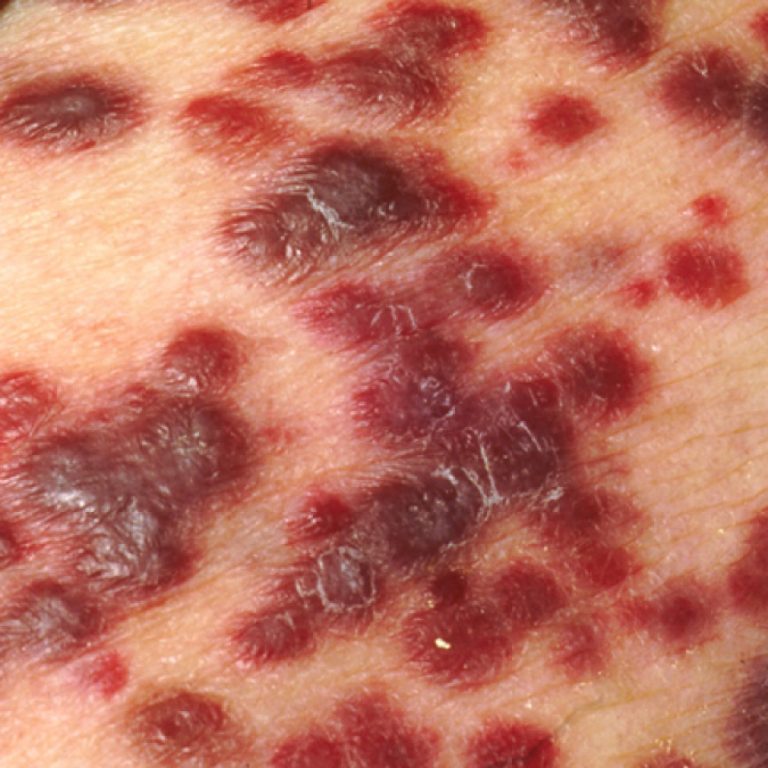
On Dec. 9, 2022, the U.S. Food and Drug Administration (FDA) approved Genentech’s atezolizumab (Tecentriq) for adult and…

On Dec. 8, 2022, Pfizer and BioNTech announced the U.S. Food and Drug Administration had granted Emergency Use…

On Dec. 8, 2022, the National Institutes of Health researchers reported that areas of the genome related to…

On Dec. 8, 2022, Moderna announced it had received emergency use authorization from the U.S. Food and Drug…
On Dec. 7, 2022, Novavax announced that Health Canada had approved a supplement to a New Drug Submission…
On Dec. 5, 2022, the Fred Hutchinson Cancer Center announced that according to a new meta-analysis women were…

On Dec. 5, 2022, Pfizer and BioNTech announced that the companies had submitted an application to the U.S….

On Dec. 2, 2022, in a multi-center collaboration led by Scripps Research, scientists announced they have achieved promising…

On Nov. 29, 2022, Novavax announced that the World Health Organization had issued an updated Emergency Use Listing…

On Nov. 22, 2022, the U.S. Food and Drug Administration approved the investigational gene therapy etranacogene dezaparvovec or…

On Nov. 18, 2022, Pfizer and BioNTech announced results from an analysis examining the immune response induced by…

On Nov. 18, 2022, Novavax announced that Health Canada had granted expanded authorization for Nuvaxovid (COVID-19 Vaccine (Recombinant…

On Nov. 18, 2022, the World Health Organisation for Animal Health (WHO) announced that Peru had reported its…

On Nov. 17, 2022, the U.S. Food and Drug Administration (FDA) approved Tzield (teplizumab-mzwv) injection to delay the…

On Nov. 16, 2022, the Cancer Prevention and Research Institute of Texas (CPRIT) approved $12 million in recruitment…

On Nov. 16, 2022, researchers at the National Institutes of Health (NIH) announced they had successfully identified differences…

On Nov. 15, 2022, Moderna reported findings from a Phase II/III clinical trial where bivalent Omicron-targeting booster candidates,…
On Nov. 14, 2022, in a study published in Nature Communications, researchers discovered the pathway by which chronic…
On Nov. 9, 2022, Novavax announced that the Medicines and Healthcare products Regulatory Agency (MHRA) in the United…
On Nov. 8, 2022, Novavax announced topline results from its Phase 3 Boosting Trial for the SARS-CoV-2 rS…
On Nov. 7, 2022, the NHS Blood and Transplant (NHSBT) reported that red blood cells grown in a…

On Nov. 5, 2022, the U.S. Department of Agriculture’s (USDA) Animal Plant Health Inspection Service (APHIS) confirmed the…

On Nov. 3, 2022, Health Canada authorized an adapted version of the Moderna Spikevax COVID-19 vaccine that targets…

On Nov. 2, 2022, Hologic announced that it had been awarded a $19 million contract from the Biomedical…
On Nov. 1, 2022, the National Institutes of Health (NIH) announced that adults with hypertension saw a small,…
On Nov. 1, 2022, the Medicines Control Authority of Zimbabwe announced that it had approved the use of…

On Oct. 31, 2022, Moderna announced that it had received approval from the Ministry of Health, Labour and…

On Oct. 27, 2022, Inovio Pharma announced that it has discontinued its internally funded efforts to develop INO-4800…