“LEQEMBI®” (Lecanemab) Approved for the Treatment of Alzheimer’s Disease in Hong Kong
In Jul. 10, 2024, Eisai and Biogen announced that the Department of Health in Hong Kong has approved…
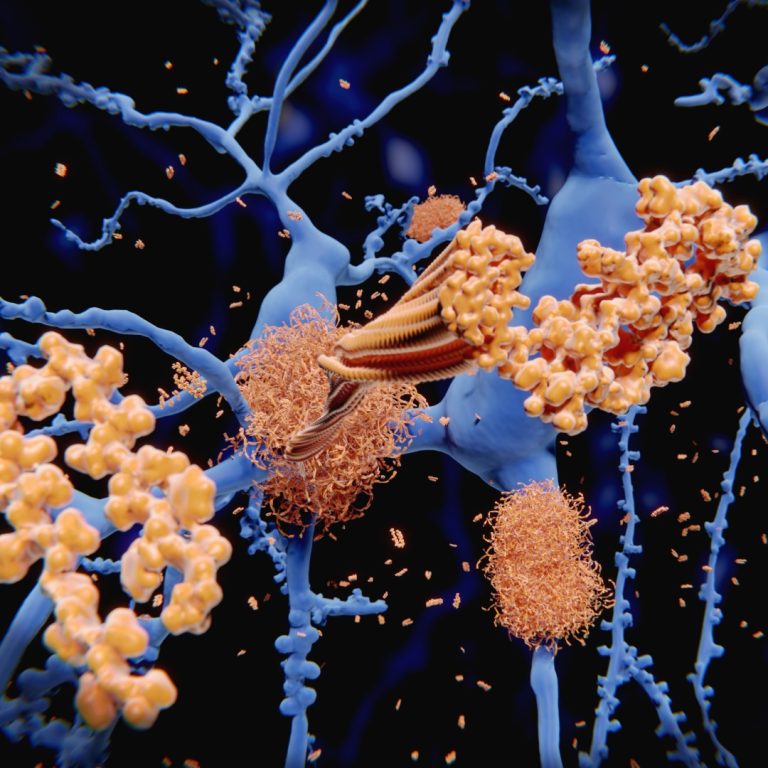
In Jul. 10, 2024, Eisai and Biogen announced that the Department of Health in Hong Kong has approved…
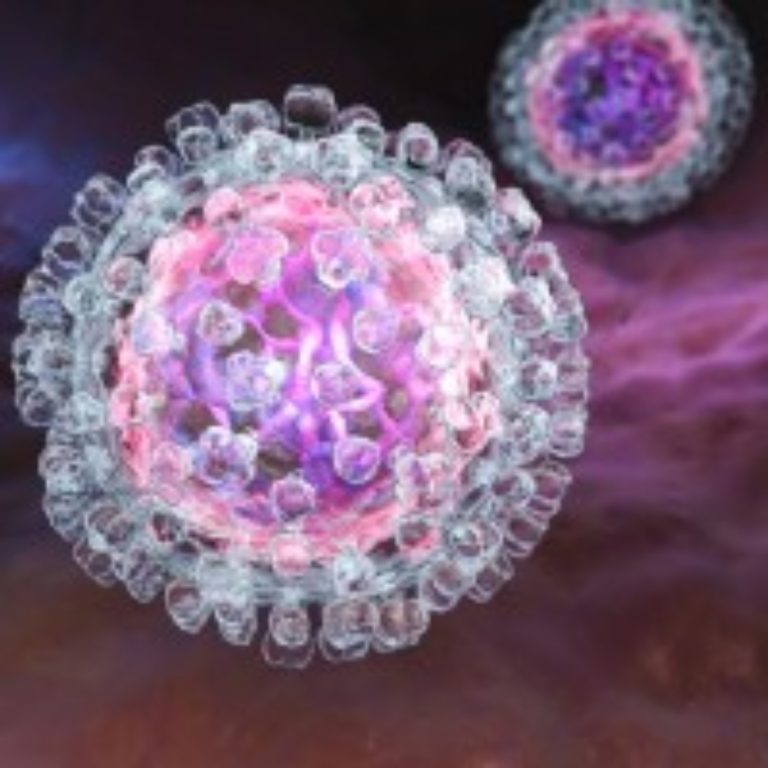
On Jul. 10, 2024, The World Health Organization (WHO) announced it had has prequalified the first hepatitis C…
On Jul. 10, 2024, Scripps Research scientists announced they have identified a group of neurons in a small…
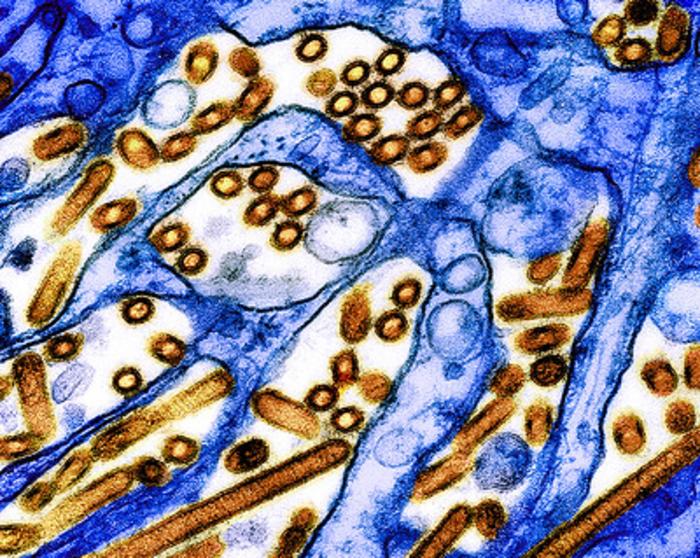
On Jul. 9, 2024, Twist Bioscience announced the expansion of its growing portfolio of synthetic viral controls with…
On Jul. 8, 2024, a study reported that babies exposed to maternal RSV (respiratory syncytial virus) vaccination in…

On Jul. 3, 2024, the Centers for Disease Control and Prevention (CDC) reported that a human case of…

On Jul. 3, 2024, Regeneron Pharmaceuticals and Sanofi announced that the European Commission (EC) has approved Dupixent® (dupilumab)…
On Jul. 2, 2024, scientists at the National Institutes of Health (NIH) have uncovered a brain circuit in…

On Jul. 2, 2024, a study was released that showed evidence that commercial tattoo and PMU inks are…
On Jul. 2, 2024, Emergent BioSolutions announced it had received more than $250 millionin contract modifications from the…

On Jul. 2, 2024, Moderna announced a project award of $176 million through the Rapid Response Partnership Vehicle…

On Jul. 1, 2024, scientists at the U.S. Department of Agriculture (USDA)’s Agricultural Research Service (ARS) and university research partners…

On Jun. 28, 2024, the California Institute for Regenerative Medicine (CIRM), one of the world’s largest institutions dedicated…
On Jun. 27, 2024, the U.S. Food and Drug Administration granted marketing authorization to Cepheid for the Xpert…

On Jun. 27, 2024, the U.S. Centers for Disease Control and Prevention’s (CDC) recommended the updated 2024-2025 COVID-19…

On Jun. 27, 2024, C2N Diagnostics (C2N), has entered into a non-exclusive agreement with Mayo Clinic Laboratories, for…
On Jun. 26, 2024, National Institute of Allergy and Infectious Diseases (NIAID) announced that research has led to…

On Jun. 26. 2024, the World Health Organization reported that new data show that nearly one third (31%)…

On Jun. 26, 2024, the U.S. Centers for Disease Control and Prevention (CDC) updated its recommendation for the…

On Jun. 25, 2024, Merck Animal Health announced U.S. Department of Agriculture (USDA) approval of NOBIVAC® NXT Canine…

On Jun. 25, 2024, Evotec announced that its Seattle-based subsidiary, Just – Evotec Biologics, was selected by the…

On Jun. 25, 2024, the Finnish Institute for Health and Welfare (THL) announced that they planned to offer…

On Jun. 24, 2024, collaborative research involving two Clemson University scientists has found that some genetic traits modern…

On Jun. 24, 2024, Novo Nordisk announced plans to invest 4.1 billion US dollars to build a second…

On Jun. 20, 2024, the U.S. Food and Drug Administration expanded the approval of Sarepta Therapeutics’ Elevidys (delandistrogene…

On Jun. 19, 2024, the Institute for Health Metrics and Evaluation (IHME) released a comprehensive new report that…

On Jun. 18, 2024, the Houston Zoo announced that Tess, a 40-year-old Asian elephant at the Zoo, had…

On Jun. 17, 2024, Merck announced that the U.S. Food and Drug Administration (FDA) had approved CAPVAXIVE™ (Pneumococcal…

On Jun. 14, 2024, the National Institute of Allergy and Infectious Diseases (NIAID, reported that the amount of…

On Jun. 13. 2024, University of Minnesota researchers announced they have successfully mapped the complete genome of the…