Scientists develop a new method to study gene function in cells and tissue
On Oct. 7, 2024, Columbia University researchers announced they had developed a new optical pooled screening approach called…
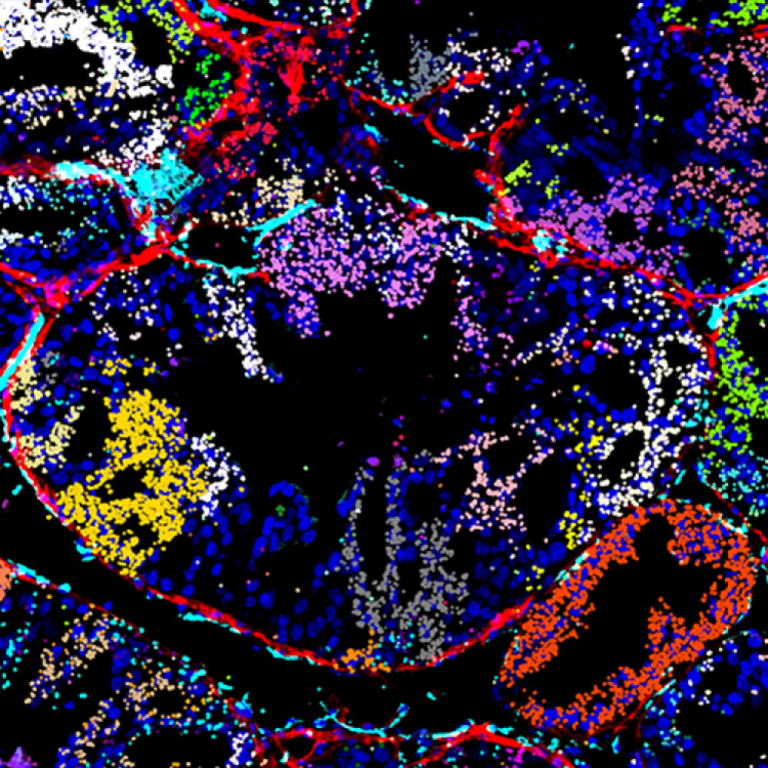
On Oct. 7, 2024, Columbia University researchers announced they had developed a new optical pooled screening approach called…

On Oct. 7, 2024, The Nobel Foundation announced that Victor Ambros from Harvard University and Gary Ruvkun from…
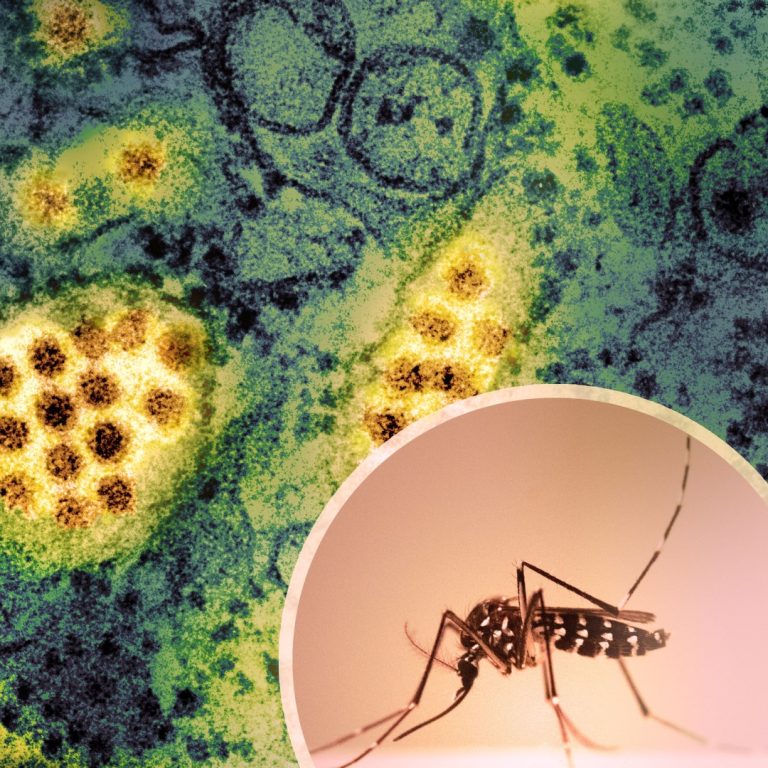
On Oct. 6, 2024, San Diego County officials announced they were investigating the first ever case of locally…
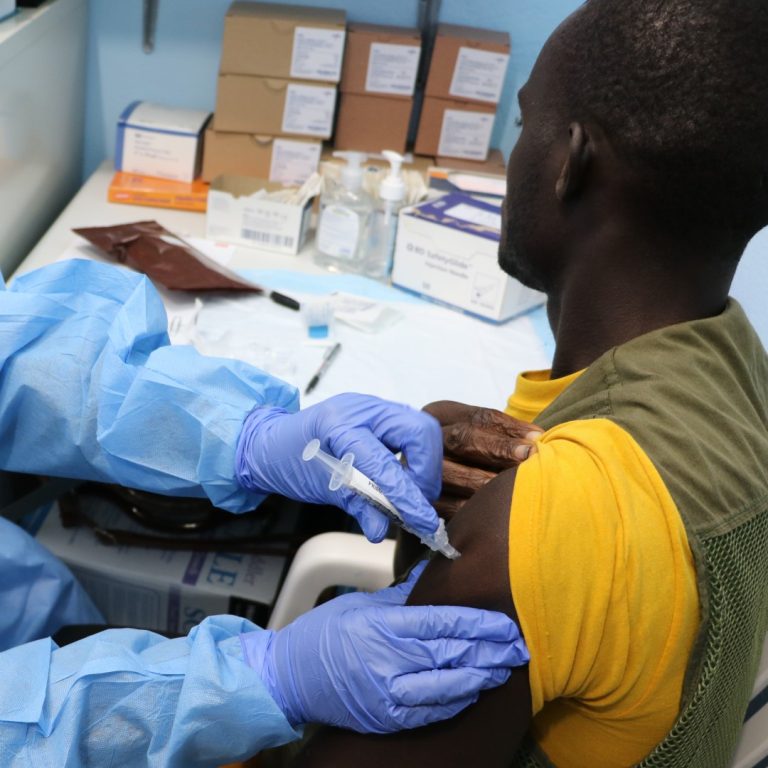
On Oct. 5, 2024, the Sabin Vaccine Institute announced it had provided its investigational Marburg vaccine to Rwanda…

On Oct. 4, 2024, researchers from Oxford university announced they had been awarded funding from Cancer Research UK…
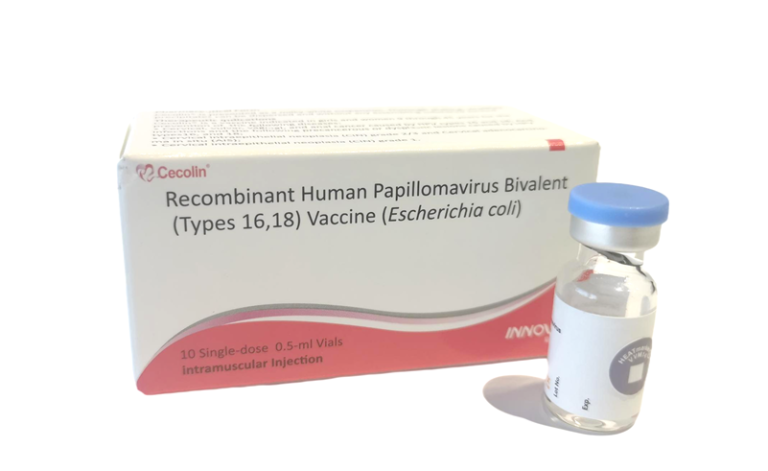
On Oct. 4, 2024, the World Health Organization (WHO) announced that a fourth WHO-prequalified human papillomavirus (HPV) vaccine…

On Oct. 4, 2024, Exact Sciences announced the U.S. Food and Drug Administration (FDA) had approved the Cologuard…

On Oct. 4, 2024, the Centers for Disease Control and Prevention (CDC) published data that showed older adults…

On Oct. 24, 2024, GSK announced an $800 million investment that will bring state-of-the-art drug substance manufacturing and…

On Oct. 4, 2024, scientists from the the University of Chile announced they had developed an injection that…

On Oct. 3, 2024, the National Health Service England (NHS) announced that Hundreds of babies have begun to…
On Oct. 3, 2024, scientists announced the largest whole-exome sequencing study of epilepsy to date, with more than…

On Oct. 3, 2024, the California Department of Public Health (CDPH) reported that the human case previously under…

On Oct. 3, 2024,the World Health Organization (WHO) launched the Global Strategic Preparedness, Readiness and Response Plan (SPRP)…
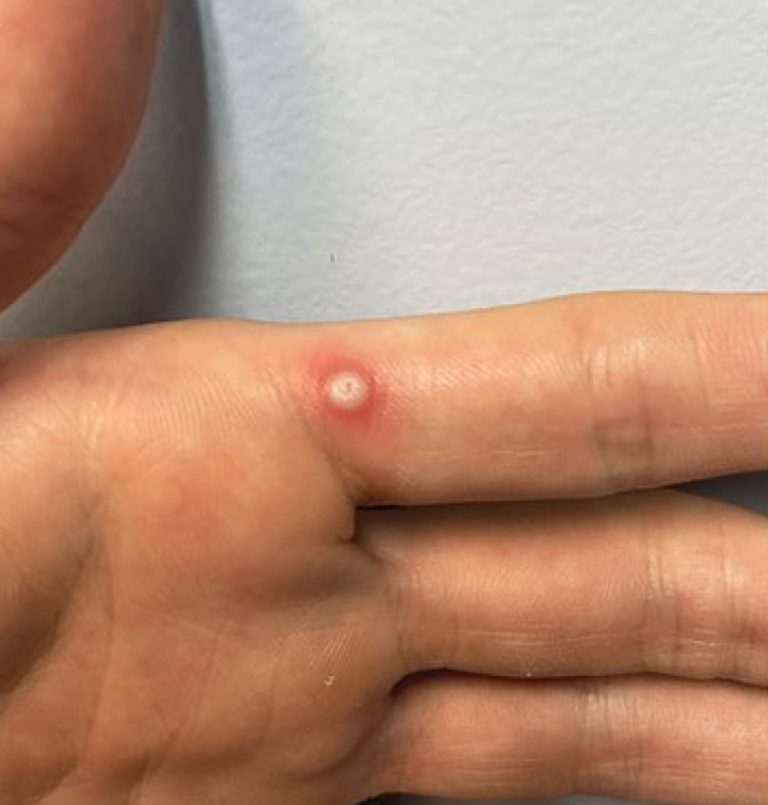
On Oct. 3, 2024, the World Health Organization (WHO) announced it had listed the first mpox in vitro…

On Oct. 3, 2024, the Nebraska Department of Health and Human Services announced that 18 cases of tularemia…

On Oct. 2, 2024, a team of researchers from the University of Tokyo announced they had discovered living…
On Oct. 2, 2024, the World Health Organization (WHO) announced it had been notified of one human case…

On Oct. 2, 2024, results from a French clinical trial have identified what experts say should now be…

On Oct. 2, 2024, scientists at Oregon State University (OSU) announced the release of two mild habanero peppers…

On Oct. 2, 2024, Eli Lilly and Company announced a $4.5 billion investment to create the Lilly Medicine…

On Oct. 1, 2024, the American Cancer Society (ACS) released Breast Cancer Statistics, 2024, the organization’s biennial update…

On Oct. 1, 2024, a study by the University of Amsterdam and the Trimbos Institute showed that 20%…

On Oct. 1, 2024, the National Academy of Medicine (NAM) announced a study showing that although the U.S….

On Sept. 30, 2024, Amneal Pharmaceuticals announced that it had launched CREXONT ® (carbidopa and levodopa) extended-release capsules…
On Sept. 28, 2024, the World Health Organization (WHO) announced that Rwandan health authorities were intensifying outbreak control…

On Sept. 27, 2024, The Golden Goose Awards were announced for the 13th annual season, which spotlights obscure, silly…
On Sept. 27, 2024, a study that evaluated the structural and functional connectivity correlates of fatigue in post-COVID…

On Sept. 27, 2024, the World Health Organization (WHO) announced the recommendations for the viral composition of influenza…
On Sept. 26. 2024, a team of scientists led by Prof. Patrik Verstreken at the VIB-KU Leuven Center…