New Mexico resident tests positive for measles after death
On Mar. 6, 2025, the New Mexico Department of Health (NMDOH) confirms that a deceased resident of Lea…
On Mar. 6, 2025, the New Mexico Department of Health (NMDOH) confirms that a deceased resident of Lea…
On Mar. 5, 2025, the World Health Organization (WHO) announced In the past two decades, tuberculosis (TB) prevention,…

On Mar. 4, 2025, Colossal Biosciences announced that its scientists have simultaneously edited seven genes in mice embryos…
On Mar. 4, 2025, researchers at a Mass Eye and Ear Institute-led trial announced taking stem cells from…

On Mar. 4, 2025, the Government of Alberta announced it had contributed $100 million in capital funding to…
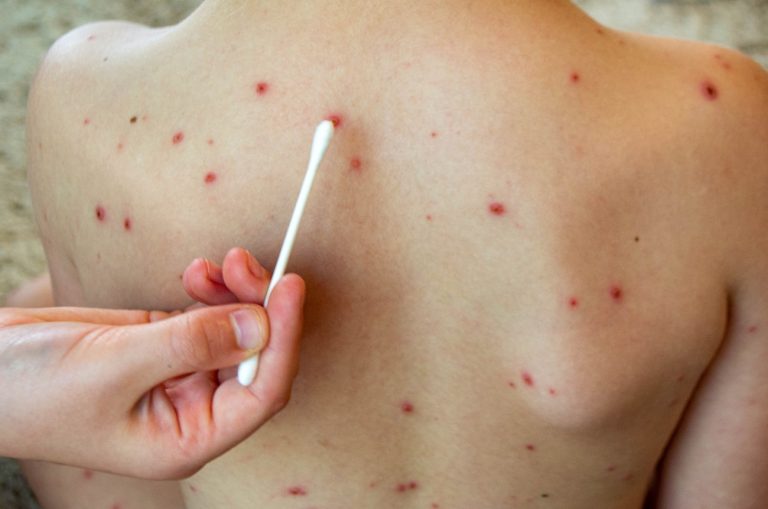
On Mar. 4, 2025, the Texas Department of State Health Services reported an outbreak of measles in the…

On Mar. 3, 2025, the Institute for Health Metrics and Evaluation reported that without urgent policy reform and…

On Mar. 3, 2025, LifeScienceHistory.com announced the release of “Earth is a Rock, Let the DNA Mold Me”…

On Mar. 3, 2025, Revvity announced the launch of EUROIMMUN’s CE-marked Anti-Measles Virus ELISA 2.0 (IgG) to support…

On Mar. 3, 2025, epidemiologists at the Texas A&M University School of Public Health have identified a link…

On Mar. 3, 2025, Genentech announced that the U.S. Food and Drug Administration (FDA) has approved TNKase® (tenecteplase),…

On Mar. 3, 2025, researchers at Fred Hutch Cancer Center announced they have discovered an overlooked mechanism driving…

On Feb. 28, 2025, the World Health Organization (WHO) announced announced the recommendations for the viral composition of…
On Feb. 27, 2025, the U.S. Centers for Disease Control and Prevention (CDC) announced that it is investigating…
On Feb. 27, 2025, a new government report adds to evidence that the HPV vaccine, once called dangerous…

On Feb. 27, 2025, ALK announced that the U.S. Food and Drug Administration (FDA) has approved ALK’s ODACTRA®…

On Feb. 27, 2025, research from Oregon State University (OSU) showed that wild birds can account for much…

On Feb. 26, 2025, the U.S. Department of Agriculture (USDA0 announced a $1 billion-dollar comprehensive strategy to curb…

On Feb. 26, 2025, the Texas Department of State Health Services reported the first death from measles in…

In Feb. 26, 2025, Eli Lilly announced that it plans to bolster its domestic medicine production across therapeutic…
On Feb. 24, 2025, the Texas Department of State Health Services reported an outbreak of measles in centered…

On Feb. 24, 2025, breast cancer is the most common cancer in women worldwide, but survival chances vary…

On Feb. 21, 2025, Novo Nordisk announced that the U.S. Food and Drug Administration (FDA) has determined the…
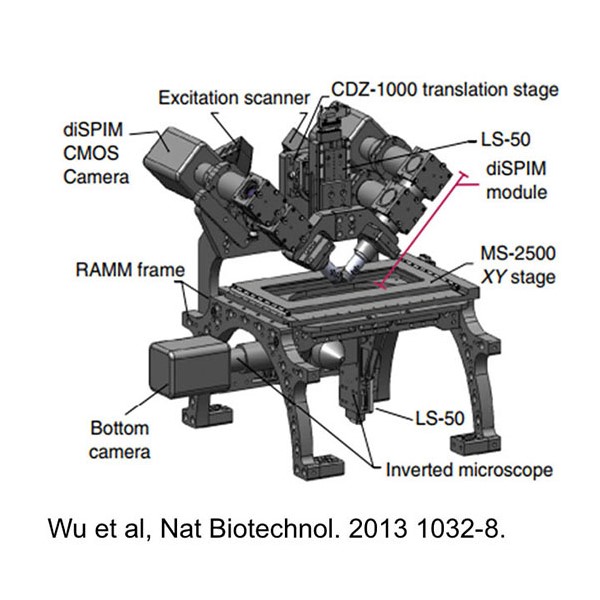
On Feb. 21, 2025, a team of researchers from the Marine Biological Laboratory (MBL) announced a hybrid custom-designed…

On Feb. 20, 2025, researchers from the University of Washington and Seattle Children’s Research Institute announced findings from…
On Feb. 19, 2025, researchers led by University of Virginia’s (UVA) Lu Q. Le, MD, PhD, announced they…

On Feb. 19, 2025, the Cancer Prevention and Research Institute of Texas (CPRIT) announced that it had approved…

On Feb. 19, 2025, Alcon announced the full U.S. commercial availability of Voyager™ DSLT, the first and only…

On Feb. 19, 2025, research from the Texas A&M College of Veterinary Medicine and Biomedical Sciences has discovered…
On Feb. 19, 2025, scientists at St. Jude Children’s Research Hospital report results from a promising new approach…