Golden Rice Approved by U.S. FDA
On May 24, 2018, the United States Food and Drug Administration (U.S. FDA) released a statement on GR2E…

On May 24, 2018, the United States Food and Drug Administration (U.S. FDA) released a statement on GR2E…

On May 21, 2018, the National Cancer Institute awarded Keck School of Medicine of USC and USC Norris…

On May 18, 2018, The Barbara Ann Karmanos Cancer Institute announced it will expand its Lawrence and Idell…

On May 18, 2018, the Boykin family pledged $10 million to support Cleveland Clinic’s Taussig Cancer Institute. Paula…
On May 16, 2018, the U.S. Centers for Disease Control and Prevention (CDC) released information about a new…
On May 14, 2018, University of California, Los Angeles (UCLA) researchers characterized the mechanism of the mammalian EAK-7…

On May 9, 2018, a new center dedicated to research on longevity and healthy aging was established at…

On May 9, 2018, the Mayo Clinic announced that it had received a $25 million gift from William…
May 4, 2018, scientists from the National Cancer Institute (NCI) reported that an analysis of a prevention clinical…
On May 2, 2018, Reed College announced that bio major Morgan Vague ’18 had isolated and bred three…

On May 2, 2018, the Stephenson Cancer Center received National Cancer Institute (NCI) designation. The Stephenson Cancer Center,…

On May 1, 2018, Keck School of Medicine of USC and nationwide partners launched a landmark $1.5 billion…

On Apr. 30, 2018, the Cancer Prevention and Research Institute of Texas (CPRIT) announced a Seed Award for…

On Apr. 27, 2018, AquaBounty Technologies announced that it had received approval from the U.S. Food and Drug…
On Apr. 23, 2018, Medigen Vaccine Biologics and GC Pharma entered an exclusive distribution agreement for GCC’s quadrivalent…
On Apr. 20, 2018, the Centers for Disease Control and Prevention (CDC) published Immunization Practices Advisory Committee (ACIP)…

Alberta Life Science Genealogy illustrates life science companies and non-profit research organizations in the Province of Alberta by…

On Apr. 11, 2018, Dr. Judith G. Hall, professor emerita of pediatrics and medical genetics at the University…

On Apr. 5, 2018, researchers funded by the National Institutes of Health (NIH) announced they had completed a…
On Apr. 3, 2018, the World Health Organization (WHO) recommended the introduction of typhoid conjugate vaccine (TCV) for…

On Apr. 1, 2018, the American College of Obstetricians and Gynecologists released a committee opinion on influenza vaccination…
On Mar. 23, 2018, University of Southern California (USC) researchers launched a massive scientific effort to construct a…
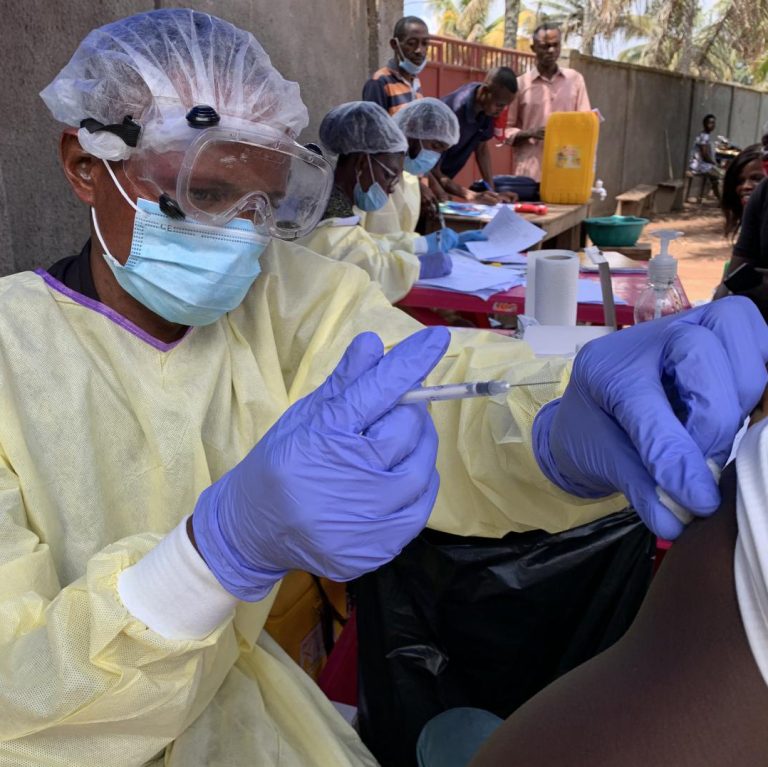
On Mar. 20, 2018, in response to the Ebola outbreak that claimed more than 10,000 lives in West…

On Mar. 15, 2018, the University of British Columbia (UBC) home for sports medicine and exercise science opened…

On Mar. 15, 2018, the University of Washington School of Medicine announced the establishment of the world’s first…

On Mar. 9, 2018, Boston-based cancer company Partner Therapeutics announced that it had acquired the global rights to…

On Mar. 9, 2018, the Keck Medicine of University of Southern California (USC) significantly expanded its patient care…

On Mar. 9, 2018, Seattle Genetics announced it had completed its acquisition of Cascadian Therapeutics for approximately $614…

On Mar. 8, 2018, Sanofi acquired Waltham, Massachusetts-based Bioverativ for for $11.6 billion. Bioverativ’s extended half-life therapies, Eloctate…

On Mar. 7, 2018, a $24-million donation to the University of British Columbia’s (UBC) faculty of medicine will…