InteliSwabᆴ COVID-19 rapid test validated to detect Omicron BA.2, BA.2.12.1, BA.3 and BA.5 subvariants
On May 17, 2022, OraSure Technologies announced that its InteliSwabᆴ COVID-19 rapid tests detect the Omicron BA.2, BA.2.12.1,…
On May 17, 2022, OraSure Technologies announced that its InteliSwabᆴ COVID-19 rapid tests detect the Omicron BA.2, BA.2.12.1,…
On May 17, 2022, Pfizer and BioNTech announced the U.S. Food and Drug Administration expanded emergency use authorization…

On May 16, 2022, working with state and federal wildlife agencies and private and public zoos across Arizona,…

On May 13, 2022, the Tabula Sapiens Consortium announced it had published a molecular reference atlas for more…

On May 13, 2022, Novavax announced the submission of a request for emergency use authorization to Taiwan’s Food…

On May 12, 2022, WHOメs COVID-19 Technology Access Pool and the Medicines Patent Pool finalized a licensing agreement…

On May 12, 2022, the U.S. National Institutes of Health announced that it had licensed 11 COVID-19 research…

On May 12, 2022, Cepheid announced it had received Emergency Use Authorization from the U.S. Food & Drug…

On May 11, 2022, Lucira Health announced that it had submitted a request to the U.S. Food and…

On May 11, 2022, Kaiser Permanente Washington Health Research Institute reported that new treatments for people at high…

On May 9, 2022, Lucira Health announced that both its COVID-19 & Flu and COVID-19 molecular tests have…
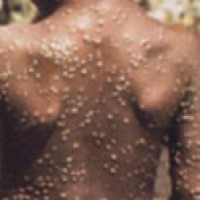
On May 7, 2022, WHO was informed of a confirmed case of monkeypox in an individual who travelled…

On May 6, 2022, the National Institute of Allergy and Infectious Diseases (NIAID) announced that it had launched…

On May 6, 2022, the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service (APHIS) confirmed the…

On May 6, 2022, Novavax announced the submission of variations to the Australian Therapeutic Goods Agency (TGA) and…
On May 6, 2022, Novavax announced that deliveries of it’s COVID-19 vaccine continued around the world and the…

On May 5, 2022, the World Health Organization (WHO) reported estimates that show the full death toll associated…

On May 4, 2022, Oxitec announced approval from the Florida Department of Agriculture and Consumer Services, including reviews…

On May 2, 2022, Sorrento announced its Phase I study of intranasal STI-9199 (COVISHIELDTM) had been fully enrolled…

On May 1, 2022, the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service confirmed the presence…

On Apr. 30, 2022, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…

On Apr. 29, 2022, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…

On Apr. 29, 2022, Moderna announced its plan to build a state-of-the-art mRNA vaccine manufacturing facility in Quebec…

On Apr. 29, 2022, Moderna announced that it had submitted for a variation to the conditional marketing authorization…

On Apr. 28, 2022, Centers for Disease Control and Prevention (CDC) reported that a person had tested positive…
On Apr. 28, 2022, the Ministry of Health of the Democratic Republic of the Congo declared an outbreak…

On Apr. 28, 2022, Moderna announced that it had submitted a request for emergency use authorization (EUA) for…

On Apr. 27, 2022, the U.S. Department of Agricultureメs Animal and Plant Health Inspection Service announced it had…

On Apr. 26, 2022, Pfizer and BioNTech submitted an application to the U.S. Food and Drug Administration (FDA)…
On Apr. 26, 2022, China’s National Health Commission reported a case of human infection with H3N8 avian influenza…