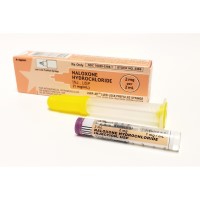
FDA approved naloxone injection to counteract opioid overdoses
On Oct. 18, 2021, the Food and Drug Administration approved ZIMHI (naloxone hydrochloride) injection as an additional option to treat opioid overdose. ZIMHI is administered using a single-dose, prefilled syringe that delivers 5 milligrams (mg) of naloxone hydrochloride solution through intramuscular (in the muscle) or subcutaneous (under the skin) injection.
FDA previously approved injectable naloxone hydrochloride products in 0.4 mg and 2 mg doses under the trade name, NARCAN.
Tags:
Source: U.S. Food and Drug Administration
Credit:
