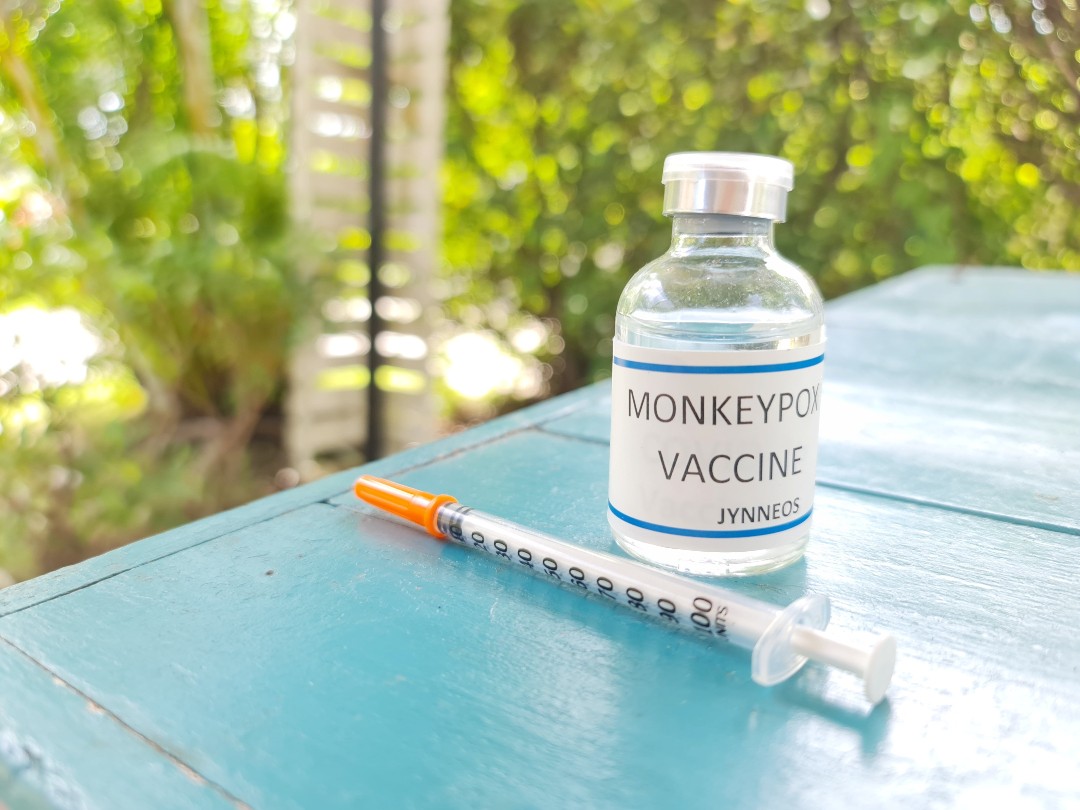
FDA approved the JYNNEOS Vaccine for the prevention of smallpox and monkeypox
On Sept. 24, 2019, the U.S. Food and Drug Administration announced approval of Jynneos Smallpox and Monkeypox Vaccine, Live, Non-Replicating, for the prevention of smallpox and monkeypox disease in adults 18 years of age and older determined to be at high risk for smallpox or monkeypox infection. This was the only currently FDA-approved vaccine for the prevention of monkeypox disease.
Jynneos does not contain the viruses that cause smallpox or monkeypox. It is made from a vaccinia virus, a virus that is closely related to, but less harmful than, variola or monkeypox viruses and can protect against both of these diseases.
Tags:
Source: U.S. Food and Drug Administration
Credit:
