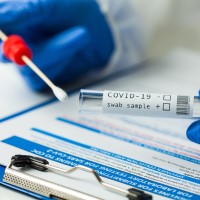
Cue Health received FDA EUA for Its rapid, portable, molecular point-of-care COVID-19 test
On Jun. 12, 2020, Cue Health announced that it had received Emergency Use Authorization from the U.S. Food and Drug Administration for the companyメs rapid, portable, point-of-care COVID-19 test. The Cue COVID-19 Test is a molecular test that detects the RNA of SARS-CoV-2, the virus that causes COVID-19, in 25 minutes using a nasal swab sample taken from the lower part of the nose.
Tags:
Source: Cue Health
Credit:
