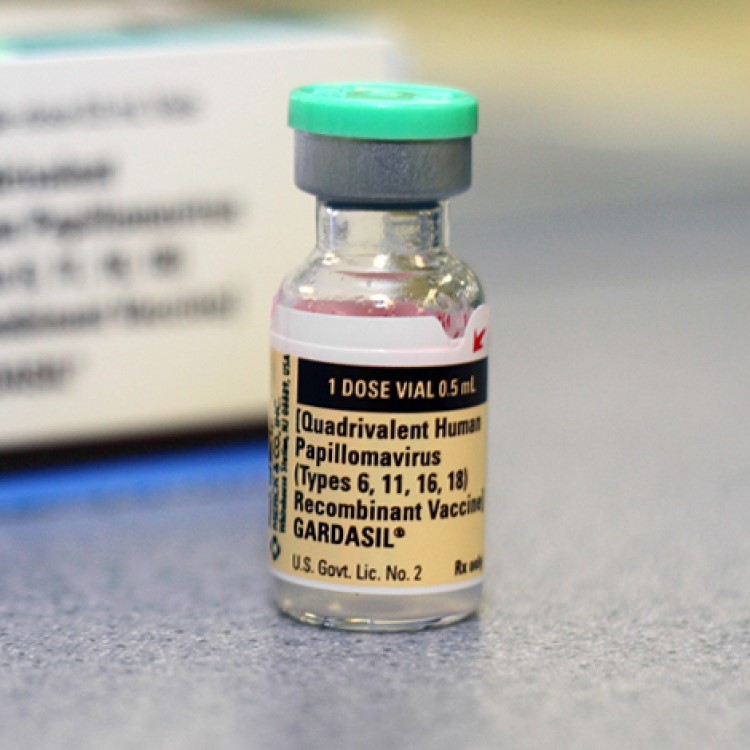
Merck recalled one batch of Gardasil, a human papillomavirus vaccine
On Dec. 16, 2013, the Centers for Disease Control and Prevention (CDC) was informed by Merck that the company planned to implement a voluntary recall of one lot (lot J007354) of Gardasil [Human Papillomavirus Quadrivalent (types 6, 11, 16, and 18) Vaccine, Recombinant], due to the potential for a small number of vials to contain glass particles as a result of breakage during the manufacturing process.
These vials were distributed between August 20, 2013, and October 9, 2013. No other lots were affected.
Tags:
Source: U.S. Centers for Disease Control and Prevention
Credit:
