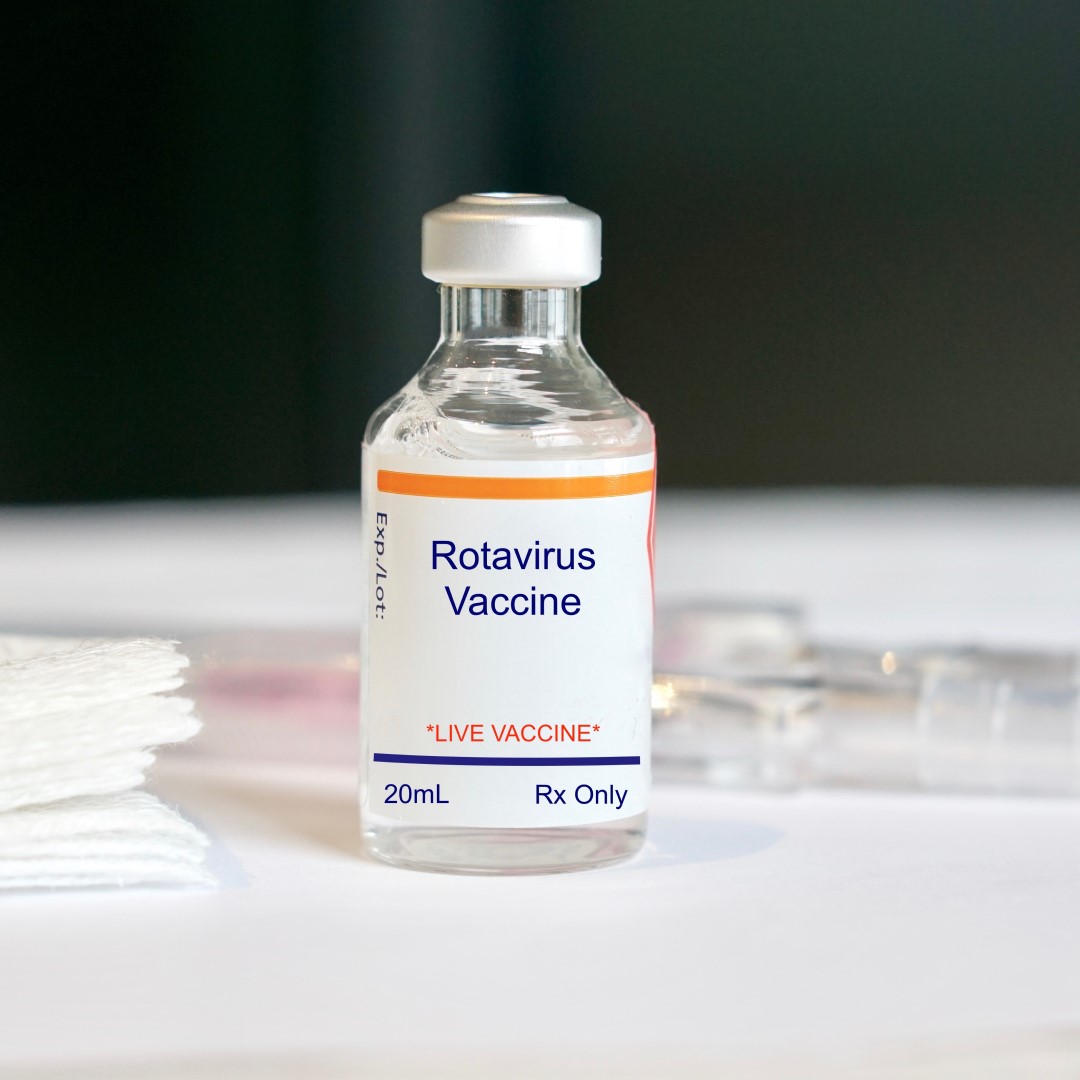
Addition of history of intussusception as a contraindication for rotavirus vaccination
On Oct. 21, 2011, the Food and Drug Administration (FDA) announced it had approved revised prescribing information and patient labeling from GlaxoSmithKline Biologicals for the monovalent rotavirus vaccine (RV1, marketed as Rotarix) and revised prescribing information and patient labeling from Merck for the pentavalent rotavirus vaccine (RV5, marketed as RotaTeq) to include history of intussusception as a contraindication.
Intussusception is a telescoping of one portion of the intestine into another, which can result in bowel obstruction and subsequent bowel ischemia. Before rotavirus vaccine was used, about 1,900 infants developed intussusception each year in the U.S.
Tags:
Source: U.S. Centers for Disease Control and Prevention
Credit:
