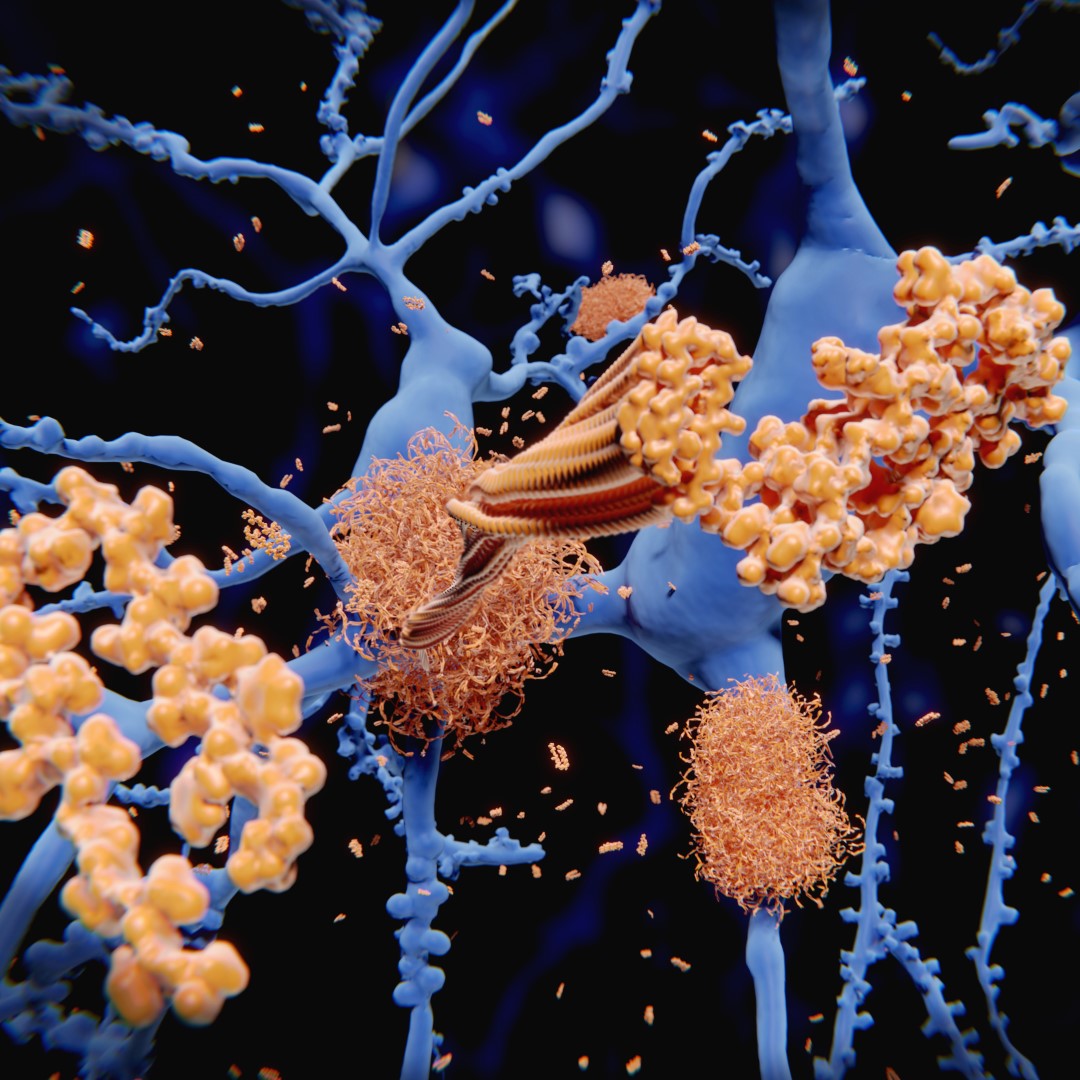
first drug approved for early Alzheimer’s disease in the UK
On Aug. 22, 2024, the Medicines and Healthcare Products Regulatory Agency (MHRA) announced it had licensed the immunotherapy drug, Lecanemab, for use in the early stages of Alzheimer’s disease in the UK, following decades of work supported by University College London (UCL) research.
Lecanemab is the first treatment of Alzheimer’s disease licensed for use in Great Britain that shows some evidence of efficacy in slowing progression of the disease.
A large-scale trial in 2022, involving 1,795 volunteers with early-stage Alzheimer’s, found that Lecanemab was able to slow cognitive decline by 27% over the course of 18 months of treatment. It also slowed down the decline in quality of life by up to 56%.
Lecanemab was fully approved by the U.S. Food and Drug Administration (FDA) as a treatment for early Alzheimer’s disease in July 2023.
The decision from the MHRA followed an application from Eisai (who make Lecanemab), made in May 2023. The approval was given as a result of expert scientific advice on the benefit risk of Lecanemab from the Commission on Human Medicines (CHM), the government’s independent advisory body.
Tags:
Source: University College London
Credit:
