Milestones in Polio
Milestones in Polio illustrates the slow but steady advancements from its clinical description and discovery to widespread infection…

Milestones in Polio illustrates the slow but steady advancements from its clinical description and discovery to widespread infection…

On Dec. 24, 2022, the U.S. Food and Drug Administration (FDA) approved updated labeling for Genentech’s capecitabine tablets…

On Dec. 23, 2022, BioNTech and Fosun Pharmaceutical announced that they had received the certificates of registration as…
On Dec. 22, 2022, resarchers at the National Institutes of Health announced they had used patient stem cells…
On Dec. 22, 2022, Gilead Sciences announced that Sunlenca (lenacapavir), in combination with other antiretroviral(s) (ARV), had been…
On Dec. 21, 2022, the U.S. Department of Health and Human Services (HHS), through the Administration for Strategic…

On Dec. 21, 2022, Roche announced that the U.S. Food and Drug Administration (FDA) had approved Actemraᆴ (tocilizumab)…

On Dec. 21, 2022, Moderna announced the the finalization of a strategic partnership with the United Kingdom (UK)…

On Dec. 20, 2022, the University of Oxford announced study results using data from 7 million people notified…

On Nov. 12, 2022, the World Health Organization (WHO) issued a Disease Outbreak News (DON) on the circulating…
On Dec. 16, 2022, Novavax announced that the Standing Committee on Vaccination (STIKO) in Germany had expanded its…

On Dec. 16, 2022, Moderna announced that the European Medicines Agency’s Committee for Medicinal Products for Human Use…

On Dec. 16, 2022, the U.S. Food and Drug Administration approved Ferring Pharmaceuticals’ Adstiladrin (nadofaragene firadenovec-vncg), a non-replicating…
On Dec. 15, 2022, the University of Oxford’s Ebola vaccine candidate has been shipped to Uganda, just 80…
On Dec. 14, 2022, the National Institutes of Health announced that two randomized, placebo-controlled trials evaluating three Ebola…

On Dec. 13, 2022, Texas Biomed announced that it had been designated as a prime contractor by the…

On Dec. 12, 2022, the Oregon Department of Agriculture and the U.S. Department of Agriculture’s Animal Plant Health…

On Dec. 11, 2022, Novavax announced that the Switzerland’s Federal Office of Public Health and Francees Haute Autorite…

On Dec. 10, 2022, CSL announced data affirming the long-term durability and safety of single-infusion HEMGENIX® (etranacogene dezaparvovec-drlb)…

On Dec. 9, 2022, the World Health Organization (WHO) announced that the first doses of one of the…
On Dec. 9, 2022, Washington University in St. Louis announced that it’s nasal COVID-19 vaccine technology had been…

On Dec. 9, 2022, Pfizer and BioNTech announced the companies had received Fast Track Designation from the U.S….
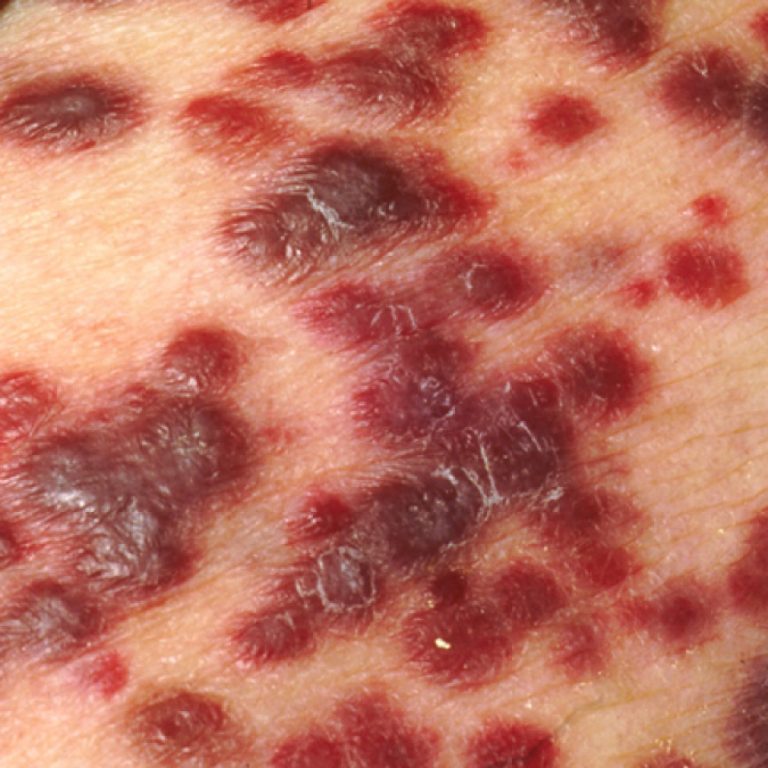
On Dec. 9, 2022, the U.S. Food and Drug Administration (FDA) approved Genentech’s atezolizumab (Tecentriq) for adult and…

On Dec. 8, 2022, Pfizer and BioNTech announced the U.S. Food and Drug Administration had granted Emergency Use…
On Dec. 8, 2022, the Department of Veterans Affairs embarked on a study to determine the most effective…

On Dec. 8, 2022, the National Institutes of Health researchers reported that areas of the genome related to…

On Dec. 8, 2022, Moderna announced it had received emergency use authorization from the U.S. Food and Drug…
On Dec. 7, 2022, Novavax announced that Health Canada had approved a supplement to a New Drug Submission…

On Dec. 7, 2022, scientists reported in Nature an ancient environmental DNA (eDNA) record describing the rich plant…
On Dec. 7, 2022, Pfizer announced that the U.S. Food and Drug Administration (FDA) had accepted for priority…