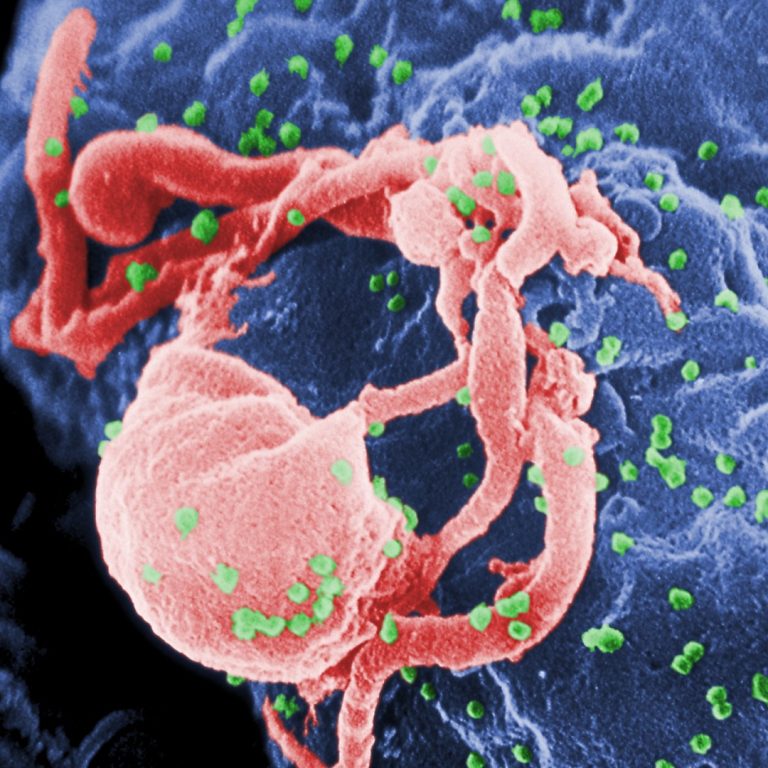
ChAdOx1 nCov-19 provided minimal protection against mild-moderate COVID-19 infection from B1351 coronavirus variant in young South African adults
On Feb. 7, 2021, Oxford University announced that an analysis, submitted as a pre-print prior to peer-review publication,…