Gilead’s Veklury (Remdesivir) associated with reduction in mortality rate in hospitalized patients with COVID-19
On Jun. 21, 2021, Gilead Sciences announced positive data from three retrospective studies of the real-world treatment of…

On Jun. 21, 2021, Gilead Sciences announced positive data from three retrospective studies of the real-world treatment of…

On Jun. 21, 2021, Cumberland Pharma released a series of case reports describing the effectiveness of Vibativ (telavancin)…

On Jun. 21, 2021, Tonix Pharmaceuticals announced that it planned to develop TNX-102 SL (cyclobenzaprine HCl sublingual tablets)…
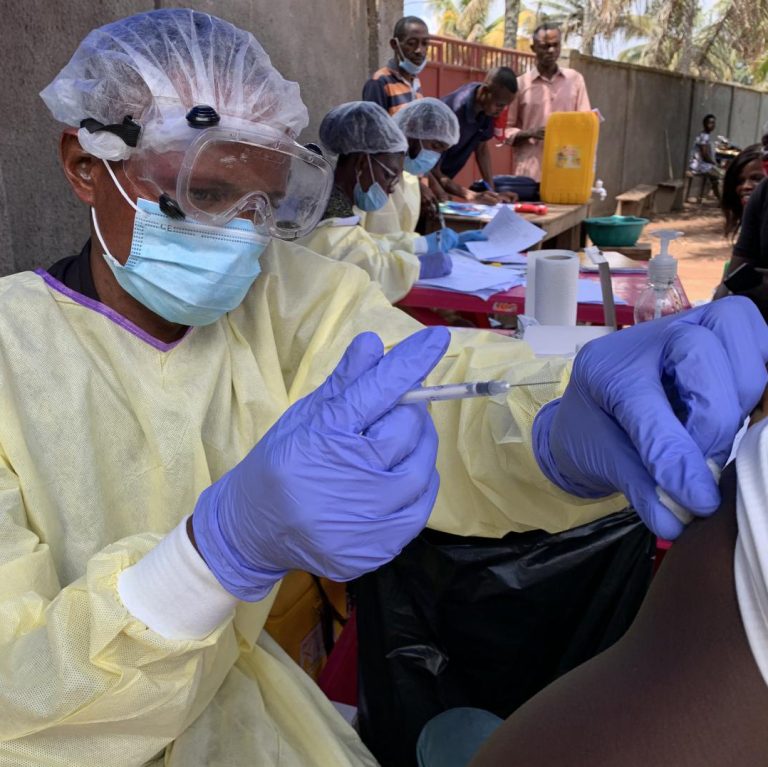
On Jun. 19, 2021, the Ebola outbreak that emerged in Guinea in mid-February was declared over. It was…

On Jun. 17, 2021, the Biden Administration is investing more than $3 billion to accelerate the discovery, development…
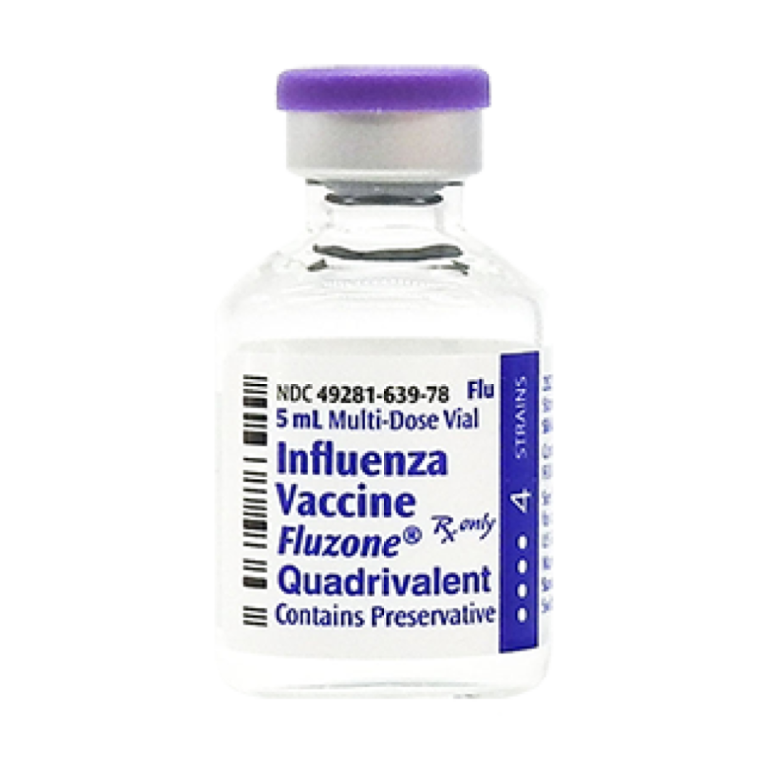
On Jun. 17, 2021, the U.S. Food and Drug Administration (FDA) Center for Biologics Evaluation and Research granted…

On Jun. 16, 2021, RELIEF THERAPEUTICS reported that its collaboration partner, NRx Pharmaceuticals, had announced additional results from…

On Jun. 16, 2021, the University of Oxford announced the Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial demonstrated…

On Jun. 16, 2021, Moderna announced that the U.S. government had purchased an additional 200 million doses of…

On Jun. 16, 2021, Regeneron announced positive results from the largest trial assessing any monoclonal antibody treatment in…

On Jun. 16, 2021, Pfizer and The Academic Research Organization (ARO) from the Hospital Israelita Albert Einstein announced…

On Jun. 16, 2021, Kaiser Permanente Washington Health Research Institute (KPWHRI) announced that was recruiting volunteers in the…

On Jun. 15, 2021, Moderna and Magenta Investments, an industrial conglomerate in the United Arab Emirates (UAE), announced…

On Jun. 15, 2021, Oncotelic announced that, as of June 11, 2021, it discontinued enrollment in its OT-101…

On Jun. 14, 2021, Novavax announced data from the first co-administration study of a SARS-CoV-2 vaccine candidate [Novavax,…

On Jun. 14, 2021, University of British Columbia researchers Dr. Jayachandran Kizhakkedathu, Dr. Caigan Du and Dr. Christopher…

On Jun. 14, 2021 Humanigen announced it had initiated a rolling review submission for Marketing Authorization (MA) by…

On Jun. 14, 2021, Moderna announced that it had submitted an authorization application to Swissmedic for use of…

On Jun. 14, 2021, Novavax announced that NVX-CoV2373, its recombinant nanoparticle protein-based COVID-19 vaccine, demonstrated 100% protection against…
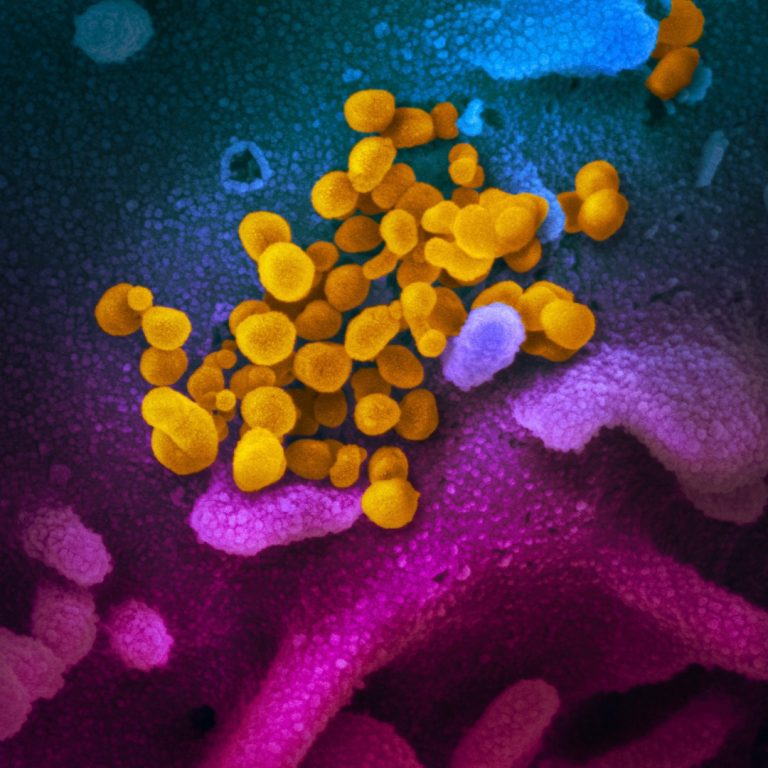
On Jun. 14, 2021, Cocrystal Pharma announced the selection of CDI-45205 as the lead compound for further development…

On Jun. 13, 2021, G7 global leaders pledged to share COVID-19 vaccine doses internationally, in support of global…

On Jun. 11, 2021, Emergent BioSolutions announced that two batches of COVID-19 vaccine manufactured by at its Baltimore…

On Jun. 11, 2021, Quidel announced it had received an amended Emergency Use Authorization (EUA) from the U.S….

On Jun. 11, 2021, The FDA issued an emergency use authorization (EUA) for the Janssen COVID-19 vaccine, two…

On Jun. 11, 2021, Novavax announced preclinical and clinical data on the company’s original recombinant protein COVID-19 vaccine…

On Jun. 11, 2021, Moderna and Tabuk Pharmaceutical announced an agreement to commercialize the Moderna COVID-19 Vaccine and…

On Jun. 10, 2021, Pfizer and BioNTech announced plans to provide the U.S. government at a not-for-profit price…

On Jun. 10, 2021, Moderna announced that it had requested an emergency use authorization (EUA) for its COVID-19…

On Jun. 9, 2021, Vertex Pharmaceuticals announced the U.S. Food and Drug Administration (FDA) approved expanded use of…

On Jun. 8, 2021, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved PREVNAR 20ル…