HDT Bio received notice to proceed from FDA for U.S. phase 1 clinical trial of RNA COVID-19 vaccine
On Jul. 1, 2021, HDT Bio announced the U.S. Food and Drug Administration had reviewed the companyメs Investigational…

On Jul. 1, 2021, HDT Bio announced the U.S. Food and Drug Administration had reviewed the companyメs Investigational…

On Jul. 1, 2021, Moderna confirmed that following the European Medicines Agency’s (EMA) Committee for Human Medicines (CHMP)…

On Jun. 30, 2021, Atea Pharmaceuticals announced positive interim results from the global Phase 2 study evaluating AT-527…
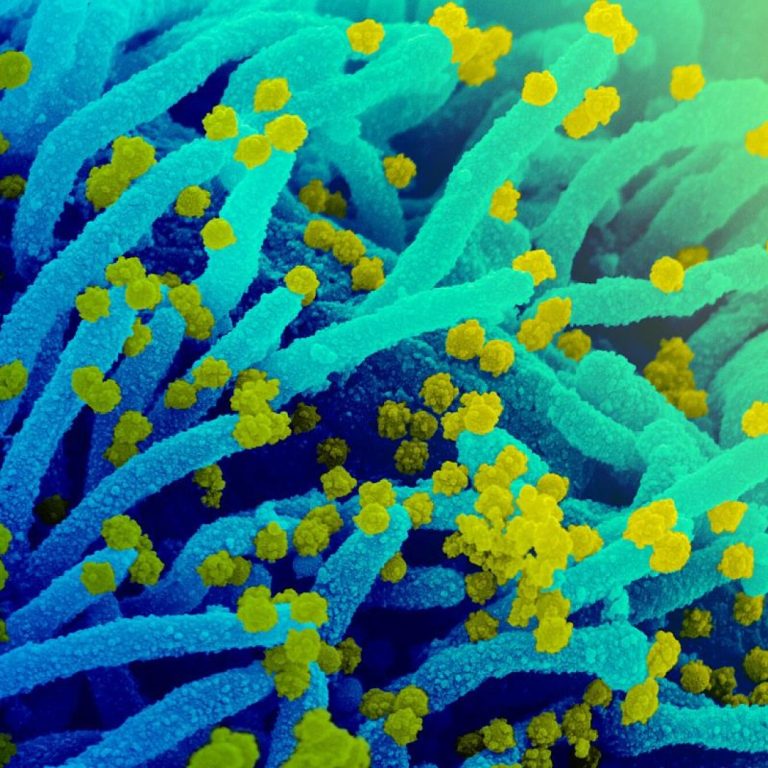
On Jun. 30, 2021, the National Institutes of Health announced a highly anticipated study that compared rapid antigen…
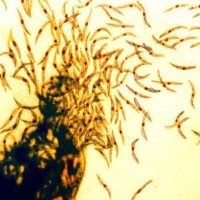
On Jun. 30, 2021, the National Institutes of Health announced that two U.S. Phase 1 clinical trials of…

On Jun. 30, 2021, following a 70-year effort, China was awarded a malaria-free certification from the World Health…

On Jun. 30, 2021, Novavax announced the publication of results from the final analysis of a pivotal Phase…
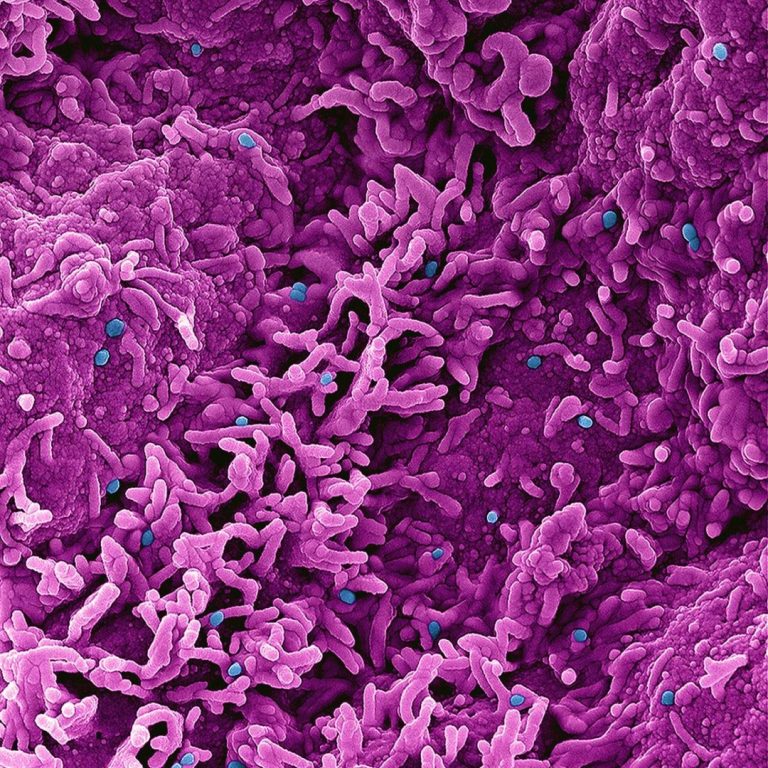
On Jun. 30, 2021, SIGA Technologies announced that it had provided TPOXX (tecovirimat) to Liverpool University Hospitals NHS…

On Jun. 30, 2021, Gavi, the Vaccine Alliance, that it had signed an advance purchase agreement with Clover…

On Jun. 30, 2021, XPhyto announced that it had signed a master supply agreement with Beovita and Tackleberries,…

On Jun. 29, 2021, Moderna announced new results from in vitro neutralization studies of sera from individuals vaccinated…

On Jun. 29, 2021, Rigel Pharma announced that fostamatinib, the Company’s novel oral spleen tyrosine kinase (SYK) inhibitor,…

On Jun. 29, 2021, the National Institutes of Health announced that an adjuvant developed with its funding had…

On Jun. 29, 2021, Paratek Pharmaceuticals announced it had delivered the first procurement of NUZYRA to the Biomedical…

On Jun. 29, 2021, Moderna announced that the government of India had issued a registration certificate and a…

On Jun. 29, 2021, Altimmune announced phase 1 AdCOVID clinical trial data that showed AdCOVID appeared to be…

On Jun. 29, 2021, VBI Vaccines announced positive Phase 1 data from its Phase 1/2 trial of the…

On Jun. 28, 2021, Meridian Bioscience announced that it had re-submitted its application for Emergency Use Authorization (EUA)…

On Jun. 28, 2021, researchers running the University of Oxford-led Com-COV study reported that alternating doses of the…

On Jun. 28, 2021, the University of Oxford in partnership with AstraZeneca began vaccinations for a new phase…
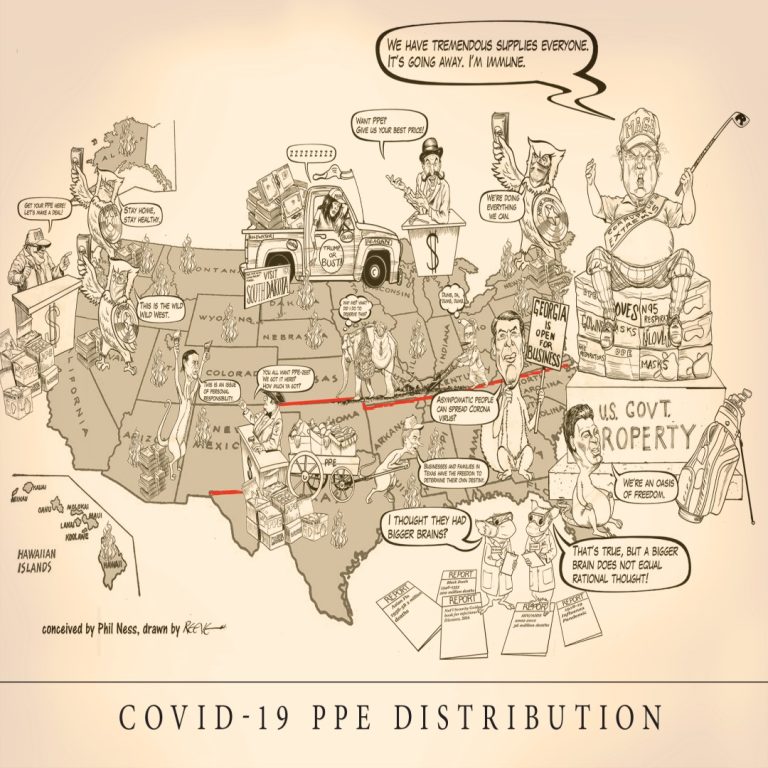
Cast of Characters: Jay Inslee, Governor, Washington | Gavin Newsom, Governor, California | Doug Ducey, Governor, Arizona |…
On Jun. 25, 2021, Roche announced that the Food and Drug Administration (FDA) has issued an Emergency Use…

On Jun. 24, 2021, researchers from the University of Oxford released their findings about the so-called ムcorrelates of…

On Jun. 23, 2021, the National Institutes of Health announced that an observational study had begun to evaluate…

On Jun. 23, 2021, Biogen and Eisai announced that the U.S. Food and Drug Administration (FDA) had granted…

On Jun. 22, 2021, the National Research Council of Canada (NRC) announced it had completed construction of the…
On Jun. 22, 2021, BioReference Laboratories, an OPKO Health company, announced a COVID-19 testing program for U.S. based…

On Jun. 22, 2021, Moderna announced that the European Commission (EC) had purchased an additional 150 million doses…

On Jun. 22, 2021, National Institutes of Health researchers reported that the prevalence of COVID-19 in the U.S….

On Oct. 6, 2021, the World Health Organization and its COVAX partners are working with a South African…